Researchers reveal how intestine microbes form metabolic methods to gas bigger brains, providing a glimpse into the evolutionary biology of primates.
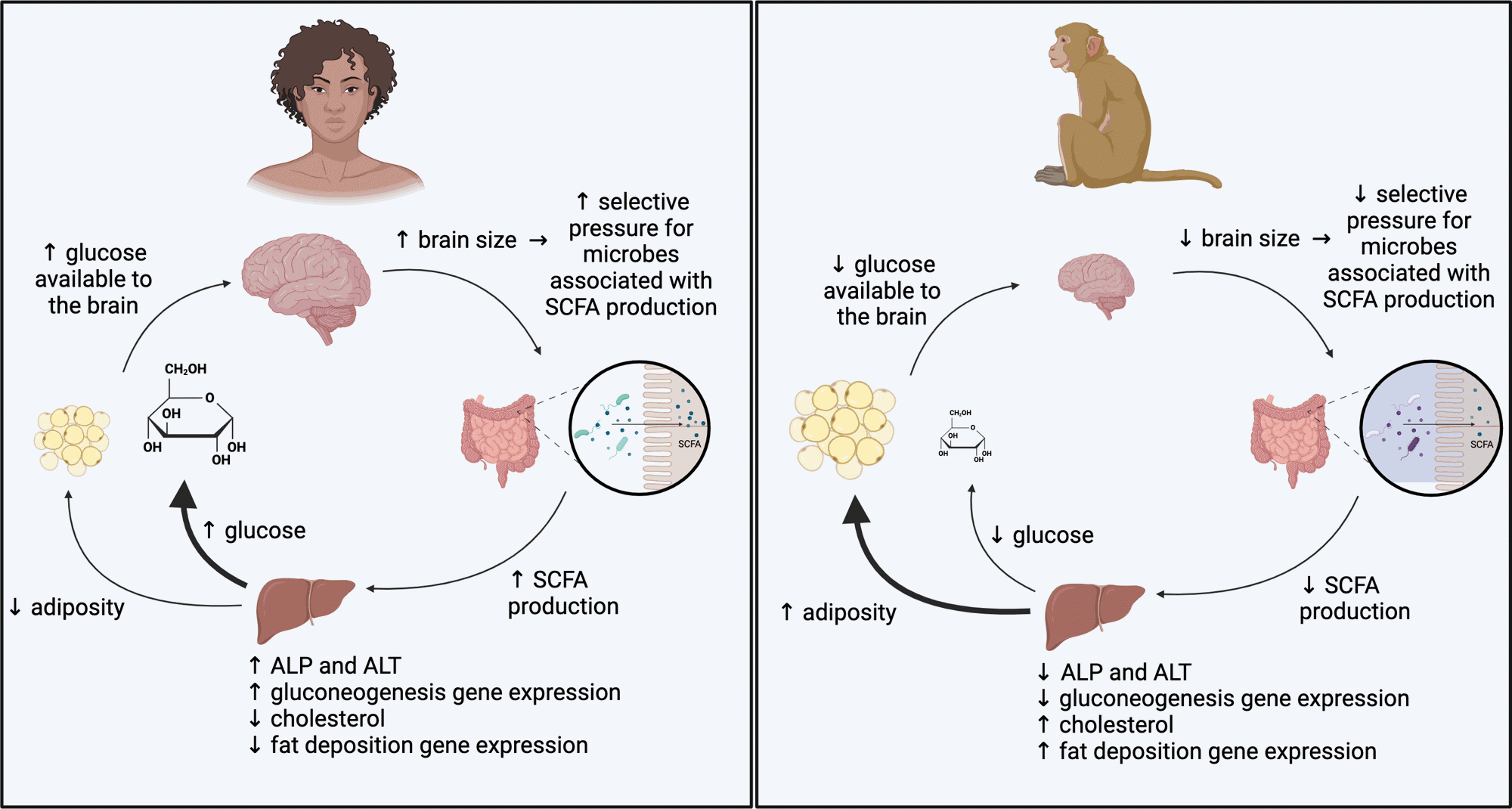 Putative mannequin for microbial influences on the metabolism of high-EQ and low-EQ primates. Our findings point out microbially mediated pathways by means of which the metabolism of high-EQ primates is biased in direction of power manufacturing and the metabolism of low-EQ primates is biased in direction of power storage.
Putative mannequin for microbial influences on the metabolism of high-EQ and low-EQ primates. Our findings point out microbially mediated pathways by means of which the metabolism of high-EQ primates is biased in direction of power manufacturing and the metabolism of low-EQ primates is biased in direction of power storage.
In a current research printed within the journal Microbial Genomics, researchers in america investigated the position of the intestine microbiome in influencing host metabolism throughout species, specializing in primates with various mind sizes. They transferred intestine microbiota from people, squirrel monkeys, and macaques to germ-free mice and examined how microbial communities contribute to metabolic traits that could be linked to mind power calls for and evolution.
Background
Giant brains are energetically expensive, particularly in primates, the place mind dimension typically correlates with elevated metabolic calls for. People, with the most important brain-to-body dimension amongst primates, present variations akin to larger glucose metabolism to maintain these energy-intensive organs. Nevertheless, the mechanisms driving such metabolic variations throughout species are poorly understood.
Present analysis has reported the involvement of genetic and epigenetic elements in these metabolic variations, however their connection to systemic metabolism stays unclear. The intestine microbiome is a crucial regulator of host metabolism and produces metabolites akin to short-chain fatty acids (SCFAs) that affect power storage, glucose manufacturing, and fats metabolism. Moreover, whereas its position in metabolic ailments, together with diabetes, is acknowledged, its contribution to interspecies metabolic variations, particularly associated to mind power calls for, is much less explored.
Concerning the Research
Within the current research, the scientists hypothesized that variations within the intestine microbiota mediate metabolic methods and stability the power wants for mind operate in opposition to these for progress and upkeep in primates with various mind sizes. The researchers performed an experiment utilizing germ-free mice to analyze how the intestine microbiome influences metabolism in hosts with totally different mind sizes.
The intestine microbiota of three primate species, people, squirrel monkeys, and macaques, was transplanted into the germ-free mice to guage the results of microbial variations on host metabolism. People and squirrel monkeys had been chosen as “brain-prioritizing” species as a result of their bigger mind sizes relative to physique dimension, whereas macaques served as a comparability with a decrease brain-to-body dimension ratio.
Fecal samples from wholesome grownup primates, freed from antibiotics, had been collected, processed, and used to inoculate germ-free mice orally on a standardized weight-reduction plan for 60 days. Weekly assessments included weight measurements, meals consumption, and metabolic evaluations, with fecal and blood samples collected for microbiome and metabolite analyses. A glucose tolerance check was administered to measure glucose regulation, and the mice underwent magnetic resonance imaging scans to evaluate physique fats distribution.
The researchers additionally used metagenomic and metabolomic analyses to determine particular microbial pathways and metabolites contributing to host metabolic traits. Excessive-resolution imaging and ribonucleic acid (RNA) sequencing of liver tissues offered insights into organ-specific metabolic responses. The microbial composition within the mouse intestine was analyzed by means of 16S ribosomal ribonucleic acid (rRNA) sequencing, whereas metagenomic methods quantified SCFA manufacturing and microbial practical pathways.
Outcomes
The outcomes confirmed that the intestine microbiome considerably influences host metabolism in methods in keeping with the mind dimension of the primate species. Mice inoculated with intestine microbiota from high-brain-to-body dimension species, akin to people and squirrel monkeys, exhibited elevated power expenditure, larger fasting glucose ranges, and enhanced gluconeogenesis. Conversely, the mice inoculated with microbiota from macaques confirmed larger fats accumulation and weight acquire.
Moreover, the mice with microbiota from species with larger mind dimension consumed extra meals however displayed decrease physique fats percentages and slower weight acquire. Elevated ranges of SCFAs, akin to acetate and propionate, had been additionally noticed in these mice, suggesting a microbial contribution to elevated glucose manufacturing and decreased fats storage.
Metagenomic evaluation revealed that microbial pathways related to power manufacturing, akin to fucose and pyruvate metabolism, had been extra considerable in high-brain-to-body dimension microbiota. Moreover, the expression of the genes related to liver operate in these mice revealed enrichment for pathways associated to power manufacturing, akin to lipid metabolism and gluconeogenesis. These modifications indicated metabolic programming geared toward prioritizing power for mind operate.
In distinction, mice inoculated with macaque microbiota exhibited microbial pathways favoring power storage. Their microbiome produced decrease SCFA concentrations and exhibited capabilities aligned with fats deposition and decreased glucose manufacturing. These variations recommended a trade-off between power allocation to the mind versus adipose tissues.
Curiously, human microbiota-inoculated mice confirmed distinctive metabolic profiles, with the best fasting glucose and propionate ranges, aligning with people’ distinctive mind power calls for. Regardless of consuming extra meals, these mice had minimal weight acquire, additional emphasizing the position of the intestine microbiota in metabolic regulation. Total, the outcomes highlighted the intestine microbiome’s means to modulate host power allocation methods, reflecting the metabolic wants of the mind dimension of the host species and the related power calls for.
Conclusions
To conclude, the research demonstrated the important position of the intestine microbiome in shaping host metabolic methods and supporting the power calls for of bigger brains in primates. The findings recommended that microbial communities affect glucose manufacturing, fats storage, and power allocation, offering insights into evolutionary variations in mind dimension.
The researchers famous that their findings present a basis for exploring how microbiomes contribute to species-specific life historical past traits, akin to progress, replica, and longevity. Future research might examine microbiome-host interactions throughout early developmental phases when mind power calls for peak.
Journal reference:
- Mallott, E. Okay., Kuthyar, S., Lee, W., Reiman, D., Jiang, H., Chitta, S., Alexandria, W. E., Layden, B. T., Sumagin, R., Manzanares, L. D., Yang, G.-Y., Luisa, M., Grey, S., Williams, L. E., Dai, Y., Curley, J. P., Haney, C. R., Liechty, E. R., Kuzawa, C. W., & Amato, Okay. R. (2024). The primate intestine microbiota contributes to interspecific variations in host metabolism. Microbial Genomics, 10, 12. DOI:10.1099/mgen.0.001322, https://www.microbiologyresearch.org/content material/journal/mgen/10.1099/mgen.0.001322
